Natural Healthy
Natural Healthy
This article introduces the relationship between oxidative stress and diseases, the selective antioxidant properties of hydrogen, and the advantages of hydrogen therapy. In the section on oxidative stress, the emphasis is placed on the types of reactive oxygen species (ROS), noting that excessive ROS can lead to tissue damage, which is a common pathological basis for many diseases. However, it is also noted that ROS at appropriate levels possess physiological functions. Many antioxidants have therapeutic potential for diseases, and hydrogen, as a new antioxidant, shares similar properties. Unlike many traditional antioxidants, hydrogen's selective antioxidant feature lies in its ability to mitigate oxidative stress without affecting the physiological functions of ROS. Moreover, hydrogen therapy is economically viable, physiologically effective, and an ideal antioxidant tool that does not disrupt bodily homeostasis.
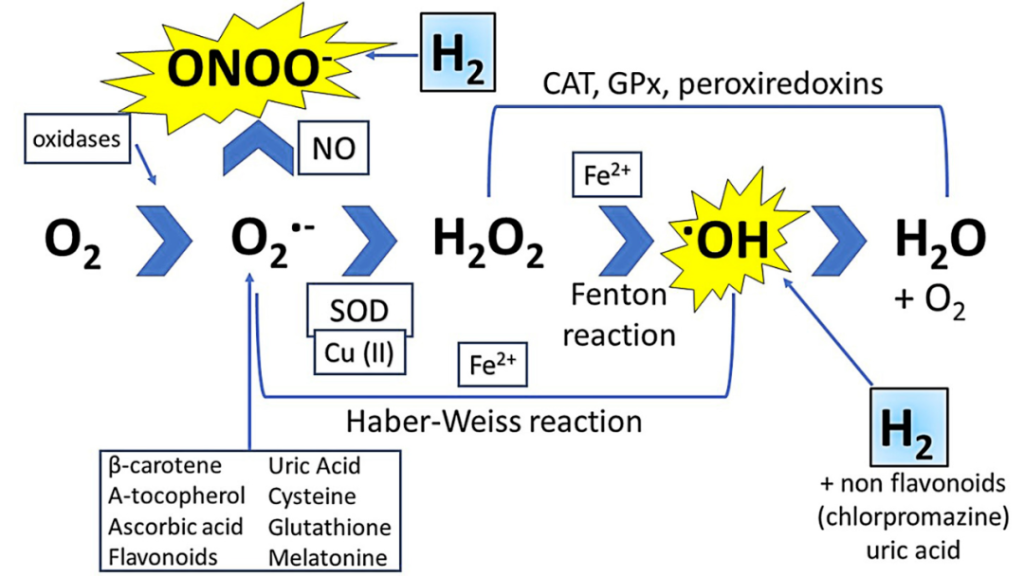
1. Oxidative stress is a common pathological basis for many diseases. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are byproducts of aerobic metabolism in living organisms. ROS include superoxide anions (O2-), hydroxyl radicals (OH), hydrogen peroxide (H2O2), and reactive nitrogen species (RNS). RNS include nitric oxide (NO), nitrogen dioxide (NO2), and peroxynitrite anions (ONOO-). These ROS/RNS have the ability to damage proteins, lipids, and nucleic acids. ROS have direct harmful effects on cellular structures such as cell membranes, mitochondria, and nuclei, and they regulate multiple cellular signaling pathways. There are various conditions where ROS overproduction occurs, such as radiation, ischemia/reperfusion (I/R) injury, inflammation, rheumatoid arthritis, and other pathologies, including aging. ROS formation can be stimulated by various external stimuli, including tumor necrosis factor-alpha (TNF-α), epidermal growth factor (EGF), interleukin-1 beta (IL-1β), hypoxia, and irradiation. Antioxidants can mitigate the harmful effects of excessive ROS. Antioxidant reserves consist of enzyme catalases (glutathione peroxidase, catalase, superoxide dismutase, etc.) and non-enzyme substances (vitamin C, vitamin E, carotenoids, etc.). Gases such as carbon monoxide (CO) and hydrogen sulfide (H2S) have been shown to be beneficial in combating oxidative stress.
ROS play important roles in many cellular functions, such as the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. ROS can activate the expression and transcriptional activity of the Nrf2 gene both in vitro and in vivo. These data suggest that adaptation to pro-oxidants reduces the activation ability of Nrf2 but simultaneously initiates a cell-enhanced antioxidant response, which depends on factors other than Nrf2. Studies have shown that excessive production of ROS and RNS or inadequate elimination thereof is one of the most important pathogenic mechanisms in many diseases, including sepsis. ROS generated by mitochondria or NADPH oxidase-4 have been shown to be crucial for receptor-mediated signal transduction through reversible oxidation of phosphatases and activation of protein kinases. In this context, cells and NADPH oxidase-4 activity intentionally producing ROS seem to play important roles in cell survival, differentiation, proliferation, and migration.
2.Hydrogen Molecule and Other Antioxidants Have the Effect of Reducing Oxidative Damage
In order to protect tissues from the effects of reactive oxygen species (ROS), research has been conducted on various antioxidant molecules. Melatonin is a well-known endogenous antioxidant and also an inhibitor of inducible nitric oxide synthase (iNOS). It also enhances the expression of endothelial nitric oxide synthase (eNOS). Melatonin has been shown to alleviate mitochondrial swelling induced by ischemia/reperfusion (I/R) and protect liver grafts from I/R injury. Ascorbic acid (AA) is an effective physiological scavenger of ROS and is often used in attempts to mitigate ROS-induced cellular damage. However, high concentrations of AA may exacerbate I/R injury, possibly due to its interaction with iron, becoming a pro-oxidant. Alpha-tocopherol (vitamin E) is a lipid-soluble antioxidant that reduces lipid peroxidation in cell membranes and lipoproteins. However, a meta-analysis suggests that high-dose vitamin E (>400 IU/day) should be avoided as it tends to increase mortality. Exercise-induced ROS production is crucial for good biological adaptation, and exogenous antioxidant supplementation can hinder the benefits of such exercise training. Another method to counteract excessive oxidative stress is to inhibit enzymes involved in ROS production. For example, matrix metalloproteinases (MMPs) are associated with oxidative stress in cardiovascular diseases. The antibiotic tetracycline family, minocycline, has been found to inhibit MMP-2 expression and protect cardiac function from I/R injury. Whether the combined use of several antioxidants and prostaglandins is more effective in enhancing organ antioxidant capacity than simply using a single chemotherapy drug remains to be observed. However, the combined use of antioxidants and stimulating organ antioxidant capacity may be effective and deserves further research.
Unfortunately, the therapeutic doses of traditional antioxidants or endogenous antioxidant inducers often exhibit high toxicity at the required levels, limiting their use to a narrow and ineffective range in preventing oxidative stress-related diseases. Molecular hydrogen, due to its unique physical and chemical properties, offers several advantages for treating diseases. In 2007, hydrogen molecules were first reported in the journal "Nature Medicine" as a selective antioxidant capable of treating oxidative stress. H2 is superior to other antioxidants because it can selectively reduce ●OH radicals while preserving other important ROS and RNS for normal cell signaling (Figure 1). New evidence suggests that hydrogen exhibits multifaceted therapeutic effects in various animal disease models and many human diseases.
3.Advantages of Hydrogen Therapy Hydrogen molecules can be ingested in various ways, including inhalation or dissolution in a solution at a concentration of 0.8 mM (1.6 mg/L), which can then be ingested as hydrogen water or in the form of hydrogen saline. This dosage is sufficient to exert biological effects in human diseases such as metabolic syndrome. The biological effects of hydrogen include antioxidation, anti-inflammation, anti-apoptosis, anti-shock, and autophagy regulation. These effects are achieved through direct scavenging of free radicals and/or indirect regulation of signal transduction and gene expression, each involving multiple signaling pathways and crosstalk. Hydrogen has high diffusibility and easily reaches subcellular compartments such as mitochondria and nuclei, which are the main sites of ROS production and DNA damage, respectively. Hydrogen can selectively reduce harmful hydroxyl radicals and peroxynitrite, but does not reduce the steady-state level of nitric oxide (NO). Lack of reactivity with NO makes hydrogen compatible with NO, which has been clinically proven effective. At therapeutic concentrations, hydrogen lacks reactivity with other gases, allowing it to be used in conjunction with other therapeutic gases, including inhaled anesthetics.